CLOCK acetylates PRPS to promote de novo nucleotide synthesis and tumor growth
The biological clock is the timing controller of life activities, maintaining the orderly coordination of life activities at the organ tissue and cell levels, and presenting a clear circadian rhythm. The disturbance of the biological clock is closely related to the occurrence and development of various diseases including cancer. In 2017, Jeffrey C. Hall, Michael Rosbash and Michael W. Young won the Nobel Prize in Physiology or Medicine for discovering the important role of circadian rhythm in physiology and disease.
The mammalian circadian clock is composed of a time-delayed transcription-translation feedback loop at the molecular level: in normal cells, CLOCK binds to BMAL1 to form a heterodimer during the day, acting as a positive regulatory element to bind to E-BOX for promoting the transcription of downstream target genes (including Per and Cry); at night, PER and CRY accumulate in the cytoplasm to form PER/CRY heterodimers, which are transferred to the nucleus and inversely inhibit the activity of CLOCK/BMAL1, thereby forming functional regulation of circadian rhythm gene transcription.
Increasing evidence has shown that compared with normal tissues, there are significant differences in the function of the biological clock and the expression of the downstream genes regulated by the biological clock in tumor tissues. However, whether cancer-promoting signals elicited by growth factor receptor activation are directly involved in the regulation of the circadian clock remains unclear.
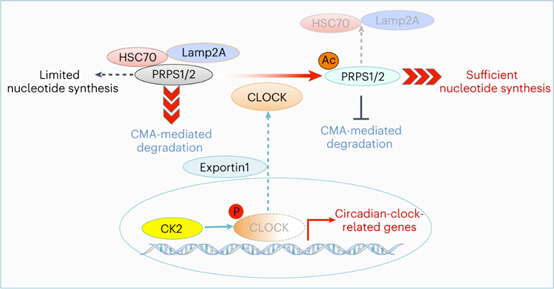
Drs Daqian Xu’ and Zhimin Lu’s groups recently reported in Nature Cell Biology (2023, PMID: 36646788) that the activation of growth factor receptors blocked the circadian clock regulation function of the transcription factor CLOCK and endowed it with an important role in regulating nucleic acid synthesis. Upon activation of insulin-like growth factor 1 receptor (IGF1R) in liver cancer cells, CK2 phosphorylates CLOCK S106, alters CLOCK conformation, and disrupts the CLOCK/BMAL1 complex, thereby inhibiting the transcription of the downstream target genes and consequently interrupting the circadian regulation of the biological clock. CLOCK S106 phosphorylation with altered structure exposes its nuclear export signal sequence (NES), leading to its translocation from the nucleus to the cytoplasm. Cytosolic CLOCK exerts the non-canonical function and functions as a protein acetyltransferase to acetylate K29 of PRPS1/2, the rate-limiting enzyme in de novo nucleic acid synthesis. PRPS1/2 K29 acetylation inhibits degradation of PRPS1/2 by blocking the HSC70 chaperon-mediated autophagy. The stabilized PRPS1/2 accelerates the de novo nucleic acid synthesis and promotes progression of liver cancer.
This study elucidates the molecular mechanism by which oncogenic signals in tumor cells interrupt the circadian clock by disruption of the CLOCK-BMAL1 dimer. Importantly, oncogenic signals confer CLOCK a vital function as protein acetyltransferase to directly control DNA/RNA synthesis. These findings have laid a foundation for the development of anti-cancer drugs by targeting the aberrantly regulated CLOCK.