Daqian Xu's and Zhimin Lu's teams discover new ferroptosis regulatory pathway
Activation of receptor protein kinases is ubiquitous in various cancers, and the impact on ferroptosis is unknown.
On May 8, 2023, Xu Daqian, Lu Zhimin of Zhejiang University published a paper entitled " Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function " in Nature Cell Biology (IF=28) research paper, which demonstrates that insulin-like growth factor 1 receptor signaling-activated AKT phosphorylates creatine kinase B (CKB) T133, reduces CKB metabolic activity, and increases CKB interaction with glutathione peroxidase 4 (GPX4 ) combination.
Importantly, CKB acts as a protein kinase to activate and phosphorylate GPX4 S104. This phosphorylation prevents the association of HSC70 with GPX4, thereby abolishing chaperone-mediated autophagy-regulated degradation of GPX4, attenuating ferroptosis, and promoting tumor growth in mice. In addition, GPX4 levels were positively correlated with CKB T133 and GPX4 S104 phosphorylation levels in human HCC specimens and correlated with poor prognosis in HCC patients. In conclusion, this study reveals a key mechanism by which tumor cells counteract ferroptosis through the nonmetabolic function of CKB to enhance GPX4 stability, and highlights the potential of targeting CKB protein kinase activity for cancer therapy.
Ferroptosis is a regulated form of cell death caused by the generation of iron-dependent reactive oxygen species (ROS) from excessive lipid peroxidation. Ferroptosis is regulated by a series of enzymatic reactions, including the biosynthesis of polyunsaturated fatty acid (PUFA)-containing phospholipids (such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, cardiolipin, etc.) Enzymatic selective oxidation of these lipids. The glutathione-dependent lipid hydroperoxidase glutathione peroxidase 4 (GPX4) utilizes glutathione (GSH) as a cofactor to convert toxic lipid hydroperoxide (L-OOH ) into nontoxic lipid alcohol (L-OH), which plays an important role in resisting irreversible peroxidation and inhibiting ferroptosis. In addition to being involved in pathological cell death associated with degenerative diseases, stroke, cerebral hemorrhage, etc., GPX4-regulated ferroptosis is an important mode of death in mammalian cancer cells. Inhibition of GPX4-induced ferroptosis has emerged as a therapeutic strategy to trigger cancer cell death. However, the mechanisms underlying the regulation of GPX4 stability in cancer cells and the impact of this regulation on tumor growth remain unclear.
Recent advances in cancer metabolism research have shown that cancer cells regulate canonical functions of metabolic enzymes to produce building blocks, metabolites, and energy required for proliferation, invasion, and metastasis. Importantly, cancer cells also confer a part-time function on metabolic enzymes in regulating many instrumental cellular activities. As metabolic enzymes, creatine kinase (CK), ATP synthase, phosphoglycerate kinase and pyruvate kinase are the key enzymes directly responsible for ATP production. CK catalyzes the reversible transfer of phosphate groups from ATP to creatine, plays a key role in regulating cellular energy homeostasis, and consists of two subunits, either B (brain type, CKB) or M (muscle type, CKM). CKB (CK-BB) is encoded by the CKB gene and expressed in brain, smooth muscle and other tissues, while CKM (CK-MM) and CK-MB are mainly expressed in skeletal muscle and cardiac muscle. Phosphoglycerate kinase and pyruvate kinase have non-canonical functions that contribute to tumor growth. Although key metabolic functions of CKB in cellular energy homeostasis have been revealed, whether CKB has a part-time function that contributes to tumor development remains unknown.
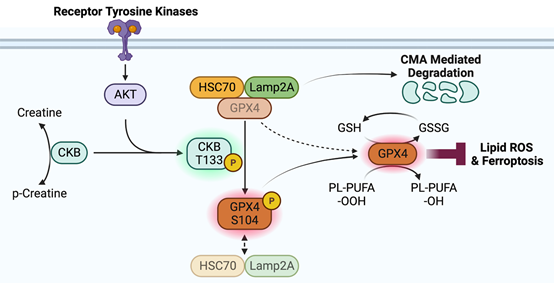
Schematic diagram of the mechanism of CKB regulating ferroptosis
This study found that IGF1R signaling activates AKT, which binds CKB and phosphorylates CKB at T133. Phosphorylated CKB reduces its association with creatine, reduces the metabolic activity of CKB, and thus acquires the ability to interact with GPX4. Importantly, GPX4-associated CKB acts as a protein kinase to phosphorylate GPX4 at S104. CKB R292H ATP-binding-deficient mutants lost the ability to phosphorylate GPX4, whereas CKB C283S produced binding-deficient mutants that did not, further suggesting that CKB uses ATP as a phosphate donor to phosphorylate GPX4. Phosphorylation of GPX4 S104, which is located near the CMA target motif in GPX4, prevents HSC70 from binding to GPX4, thereby abolishing CMA-mediated degradation of GPX4. IGF1 treatment attenuated cystine deprivation and erastin-induced decreased lipid ROS generation and cell death; whereas expression of CKB T133A or GPX4 S104A abolished this effect. Furthermore, expression of CKB T133A or GPX4 S104A reduced tumor growth and greatly exacerbated the inhibition of ferroptosis and tumor growth.
In conclusion, this study demonstrates that insulin-like growth factor 1 (IGF1) receptor (IGF1R) activation in hepatocellular carcinoma (HCC) cells increases the expression of GPX4, which is dependent on the activity of CKB protein kinase. CKB phosphorylates GPX4, thereby preventing its degradation, counteracting ferroptosis in HCC cells and promoting tumor growth.


